Rank these items from most acidic to least acidic – Delving into the fascinating realm of acid-base chemistry, this guide embarks on a journey to unravel the intricacies of acidity, empowering readers with a comprehensive understanding of the ranking of acids from most acidic to least acidic. Embarking on this scientific exploration, we unravel the fundamental concepts that govern acid-base interactions, illuminating the factors that determine the strength and acidity of various substances.
As we delve deeper into this realm of chemistry, we will encounter the Brønsted-Lowry theory, the cornerstone of our understanding of acids and bases, and explore the pH scale, a crucial tool in gauging acidity. Furthermore, we will delve into the concept of acid strength, examining the factors that influence the ionization constant (Ka) and ultimately dictate the potency of an acid.
Acid-Base Concepts

Acids and bases are fundamental chemical concepts that play a crucial role in various scientific disciplines and everyday life. Understanding their properties and behavior is essential for comprehending chemical reactions and their applications.
According to the Brønsted-Lowry theory, acids are substances that donate protons (H +ions), while bases are substances that accept protons.
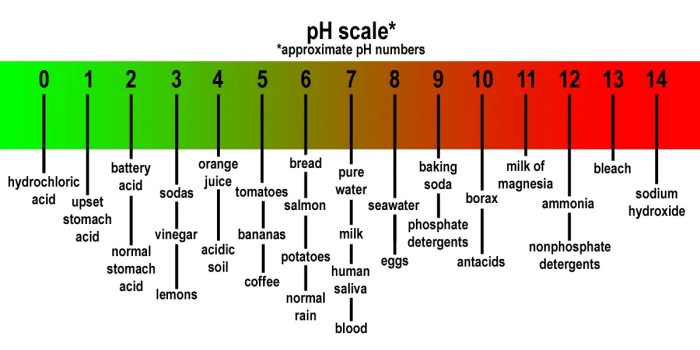
The pH scale, ranging from 0 to 14, is a measure of acidity or alkalinity of a solution. A pH of 7 is neutral, values below 7 indicate acidity, and values above 7 indicate alkalinity.
Ranking Acidity: Rank These Items From Most Acidic To Least Acidic

The strength of an acid is determined by its ability to ionize and release protons. Acids with higher ionization constants (Ka) are stronger acids and ionize more readily.
Factors that affect the strength of an acid include the electronegativity of the atom bonded to the hydrogen atom, the size of the atom, and the presence of resonance or inductive effects.
| Acid | Formula | Ka | Strength |
|---|---|---|---|
| Hydrochloric acid | HCl | 10-7 | Strong |
| Sulfuric acid | H2SO4 | 10-3 | Strong |
| Acetic acid | CH3COOH | 10-5 | Weak |
Examples of Acids

Common acids include:
- Hydrochloric acid (HCl): A strong acid used in industrial processes and laboratory experiments.
- Sulfuric acid (H 2SO 4): A strong acid used in batteries, fertilizers, and petroleum refining.
- Acetic acid (CH 3COOH): A weak acid found in vinegar and used as a food preservative.
Weak acids:
- Carbonic acid (H 2CO 3): A weak acid produced when carbon dioxide dissolves in water.
- Citric acid (C 6H 8O 7): A weak acid found in citrus fruits and used as a flavoring agent.
Applications of Acid-Base Chemistry
Acid-base chemistry has numerous applications in various fields:
| Application | Example |
|---|---|
| Food preservation | Vinegar (acetic acid) is used to preserve food by inhibiting the growth of bacteria. |
| Medicine | Antacids (bases) are used to neutralize stomach acid and relieve heartburn. |
| Industrial processes | Hydrochloric acid is used in the production of plastics, dyes, and fertilizers. |
Question Bank
What is the pH scale?
The pH scale is a logarithmic scale used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating alkalinity.
What factors affect the strength of an acid?
The strength of an acid is primarily determined by its ionization constant (Ka). A higher Ka value indicates a stronger acid, as it readily donates protons (H+ ions) in solution.
What are some examples of common acids?
Common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).