Put the following in order of increasing standard molar entropy. – Put the following in order of increasing standard molar entropy: solids, liquids, and gases. Entropy is a measure of the disorder of a system, and it is directly related to the number of possible arrangements of the molecules in the system.
Solids have the lowest entropy because their molecules are tightly packed together in a regular arrangement. Liquids have a higher entropy than solids because their molecules are more loosely packed and have more freedom to move around. Gases have the highest entropy because their molecules are completely free to move around and occupy the entire volume of the container.
The standard molar entropy of a substance is the entropy of one mole of the substance at a pressure of 1 bar and a temperature of 298.15 K. The standard molar entropy of a substance can be used to predict the spontaneity of a reaction.
A reaction is spontaneous if the change in entropy of the system is positive. The standard molar entropy of a substance can also be used to calculate the equilibrium constant for a reaction.
Overview of Standard Molar Entropy: Put The Following In Order Of Increasing Standard Molar Entropy.
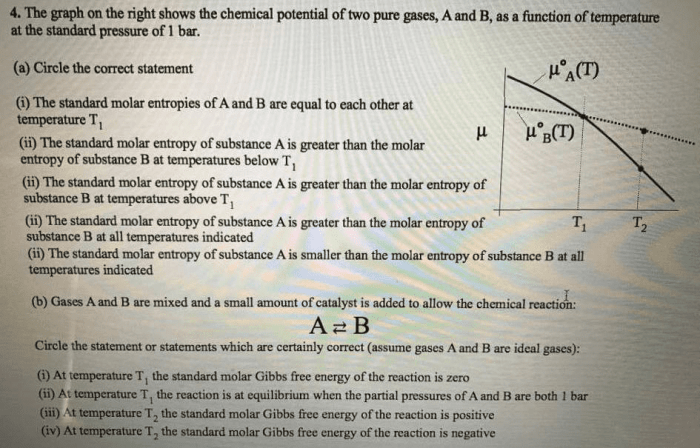
Standard molar entropy ( So) quantifies the degree of disorder or randomness within a system. It is a key thermodynamic property that helps predict the spontaneity of reactions and understand the behavior of substances under various conditions.
Standard molar entropy is measured in joules per mole Kelvin (J mol -1K -1) and represents the entropy of a substance in its standard state, which is defined as 1 mol of the substance at a pressure of 1 bar and a temperature of 298.15 K (25 °C).
The standard molar entropy of a substance is a measure of its thermal energy distribution and the number of possible microstates available to its molecules. A higher standard molar entropy indicates a more disordered system with a greater number of possible microstates.
Factors Affecting Standard Molar Entropy
- Molecular structure:Molecules with more complex structures and more degrees of freedom have higher standard molar entropy values.
- Temperature:Standard molar entropy generally increases with increasing temperature as molecules gain more thermal energy and become more disordered.
- Pressure:Standard molar entropy typically decreases with increasing pressure as molecules are forced closer together, reducing the number of possible microstates.
Methods for Determining Standard Molar Entropy, Put the following in order of increasing standard molar entropy.
Standard molar entropy can be determined experimentally using various techniques:
- Calorimetry:By measuring the heat absorbed or released during phase transitions or chemical reactions.
- Spectroscopic techniques:By analyzing the vibrational and rotational spectra of molecules.
- Computational methods:By using statistical mechanics and molecular simulations to estimate the number of microstates available to a system.
Examples of Standard Molar Entropy Values
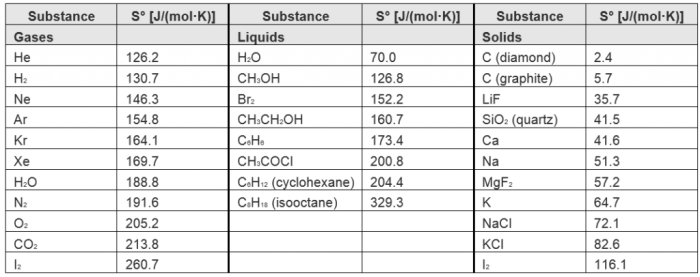
The standard molar entropy values for various substances can vary significantly:
| Substance | Standard Molar Entropy (J mol-1 K-1) |
|---|---|
| Water (liquid) | 69.95 |
| Carbon dioxide (gas) | 213.6 |
| Sodium chloride (solid) | 72.1 |
| Methane (gas) | 186.2 |
| Diamond (solid) | 2.38 |
Applications of Standard Molar Entropy
Standard molar entropy is a valuable tool in thermodynamics and has numerous applications:
- Predicting spontaneity of reactions:The change in standard molar entropy during a reaction can help predict its spontaneity.
- Equilibrium calculations:Standard molar entropy is used to calculate equilibrium constants and predict the direction of reactions.
- Understanding phase transitions:Standard molar entropy can provide insights into the disorder-order transitions that occur during phase changes.
FAQ
What is entropy?
Entropy is a measure of the disorder of a system.
What is the standard molar entropy?
The standard molar entropy is the entropy of one mole of a substance at a pressure of 1 bar and a temperature of 298.15 K.
How can entropy be used to predict the spontaneity of a reaction?
A reaction is spontaneous if the change in entropy of the system is positive.